
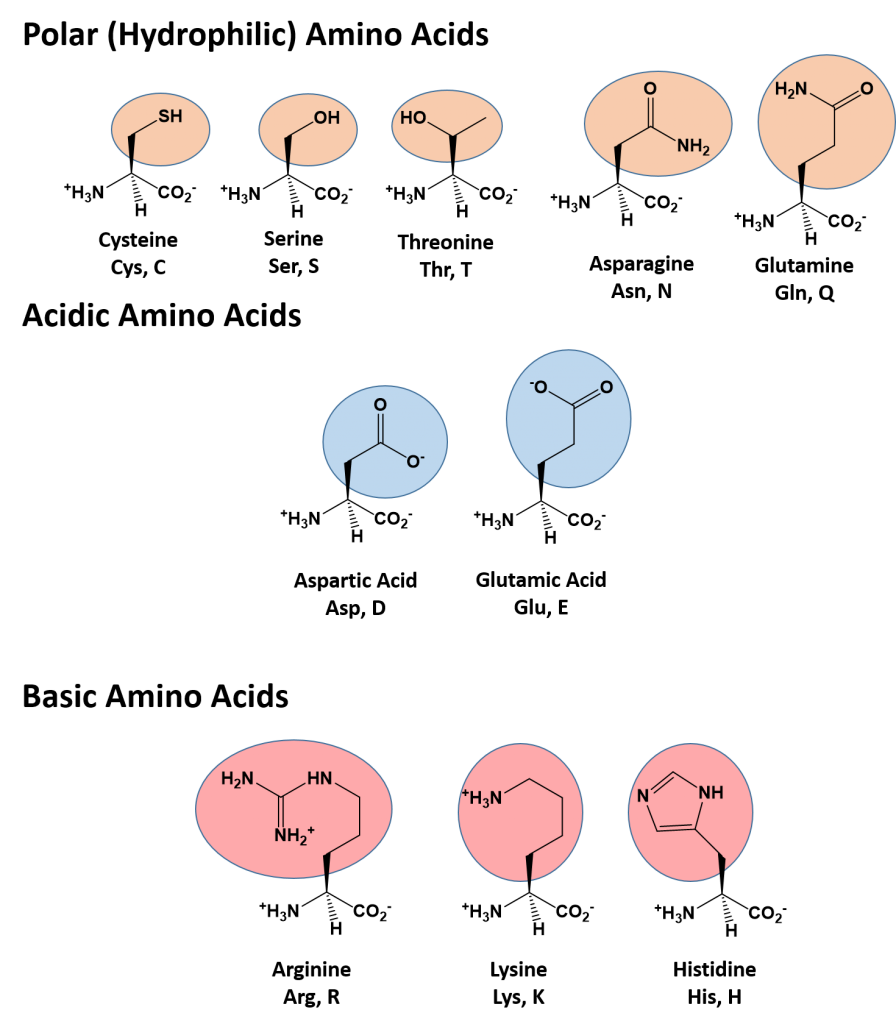
Generally considered as an asymmetrical catalyst in numerous protein synthesis, Harvard University researchers often referred to proline as the 'simplest enzyme,' which was then elaborated as proline being one the few catalysts enabling prebiotic evolution.Ĭonsidered highly unusual for an amino acid to be cyclic in its structure (because of the secondary amine), proline forms a peptide bond that does not contain hydrogen on the α amino group. Willstätter came up with the D, L-racemate synthesized from N-methylproline. It was in 1900 that Nobel-laureate Richard M. The proteins synthesized from proline also have discrete secondary structures and, therefore, appear different from that of the proteins synthesized from open-chain proteins. The proline contains a secondary amine group (the only natural amino acid having a secondary amine), giving its unique helix rings in the structure. The primary amine present on the carbon of the glutamate semialdehyde generally forms a Schiff base from which the aldehyde reduces, thus generating proline. It is also a part of the twenty most crucial amino acids as humans and other animals biosynthesize it.
Also known as L-proline, it is an imino acid or a molecule that comprises both the carboxyl and imine functional groups.


 0 kommentar(er)
0 kommentar(er)
